Why Is Infrastructure Performance Not Assessed
The purpose of performance assessment is to demonstrate fitness for use. But in the case of infrastructure, since these are the supporting business processes whose performance will be tested, infrastructure performance is indirectly verified during the validation of computerized business systems. The GAMP® Guide states that infrastructure must be qualified to be considered compliant.
Infrastructure qualification must demonstrate its ability to maintain information security.
Requirements/user requirements
In this guide, the terms user requirements and user requirement specifications will be considered equivalent, using the acronym URS.
Computer Systems Validation Jobs
-
10TypesPhiladelphia, PA - 10Types has on-going needs for for ComputerSystemsValidation professionals for on-site work in the Philadelphia and Boston areas.
| Karuna TherapeuticsRemote |
| Remote in Miami Lakes, FL 33014Full-time |
| $87.9K – $111K a year Full-timeDay shift |
| GXPeopleNew Hampshire |
| GXPeopleUnited States |
| $87.6K – $111K a year Full-time |
| $72 – $83 an hourOn call |
How Are Computerized Systems Characterized
The characterization of computerized systems is an important activity that precedes the development of a validation study. This characterization allows us to understand two fundamental aspects:
- The computerized system
- The process that serves the system
Characterization, depending on the stage of the life cycle the system is in, can be based on user requirements or on existing system specifications and process knowledge .
In some cases, a computer system can even be defined as the union of a host with other so-called satellites that complement the main functions of the system.
Among the elements to look at the characterization of the computer system are the following:
- Scope and elements of which the system is composed
- Objectives, main features and expected results
- General requirements related to the computerized system
- Support for the documentation process
Scope and definition of the system
Recommended Reading: How To Tell An Interview Candidate No
What Is 21 Cfr Part 11
Title 21 CFR Part 11 of the Code of Federal Regulations deals with the Food and Drug Administration guidelines on electronic records and electronic signatures in the United States. Part 11, as it iscommonly called, defines the criteria under which electronic records and electronic signatures areconsidered to be trustworthy, reliable and equivalent to paper records
What Is The Full Form Of Wwe What Is Its Usage

WWW is an acronym that stands for World Wide Web. It is a method of accessing information using the Internet. WWW is commonly known as the Web. It is an information system where Uniform Resource Locators identify documents and other web resources , such as https://abc.com/), interlinked by hyperlinks. We can access it over the Internet. These resources are published by a software application called a web server. The resources of the WWW are transferred via the Hypertext Transfer Protocol , and a user can access it by a software application called a web browser. The World Wide Web is built on top of the Internet, which pre-dated the Web by over two decades.
You May Like: Indeed Software Engineer Interview Questions
Frequently Asked Questions About Validation Plans
Q: What is the definition of Validation Plan?A: The FDA uses the NIST definition: A management document describing the approach taken for a project. The plan typically describes work to be done, resources required, methods to be used, configuration management and quality assurance procedures to be followed, schedules to be met, project organization, etc. Project in this context is a generic term. Some projects may also need integration plans, security plans, test plans, quality assurance plans, etc. In practice, the validation plan describes how the validation project is going to be performed.
Q: Can I see an example of a validation plan?A: We have a sample validation plan available for download.
–
Templates And Validation Examples
Templates are useful to effectively follow and document validation tasks and results. Validation examples help to get adequate information on how to conduct validation and to prepare deliverables. Labcompliance has templates and examples for validation tasks. They are indicated by E-Numbers in the list below and are either included in the Computer System Validation Package: or can be ordered from the labcompliance Examples website.
Such documentation can include templates/examples for:
Also Check: How To Prepare For Facebook Product Manager Interview
What Is The Responsibility Of The Provider
Providers play an important role during the system life cycle and each of the selected stages of the V-model.
We can classify them as follows:
- Provider of computer system and consumables
- Provider of infrastructure
- Service provider qualification and validation
To reduce the possibility of any inconvenience with any provider, you must carry a rating and approval of this before purchasing supplies or services. Also, a way to eliminate risks associated with suppliers is to ensure that the UK, is established from the same purchase order.
Suppliers responsibilities are defined in terms of the activities for which they are hired and the criticality of their participation in the process.
What Are Your Thoughts On Risk
The interviewer is asking the validation engineer for their thoughts on risk-based approach to validation because it is an important part of the validation process. Risk-based approach to validation helps ensure that the products being validated are safe and effective for their intended use. It is important to have a robust validation process in place to ensure that products meet all safety and efficacy requirements.
Example: I think that risk-based approach to validation is a great way to prioritize validation activities. By identifying and assessing risks, we can focus our efforts on the areas that are most likely to impact product quality and patient safety. This approach can help us to be more efficient and effective in our validation efforts.
Recommended Reading: What Are My Weaknesses Job Interview
Be Prepared For Compliance Audits
- Audit Checklists Ofni Systems can develop tools to facilitate your system review and ensure that your systems meet all applicable regulations.
- Gap Analysis Before an FDA inspection or regulatory compliance audit, Ofni Systems can review your internal and external quality systems against established industry and regulatory standards and assess if your companys actual practices meet those standards.
- Remediation Plans If gap analysis identifies gaps, we can create a remediation plan to address the findings.
- Compliance Training Ofni Systems can also help training your employees to be proactive in dealing with compliance issues.
What Is Deep Learning In Computer Science
Deep learning is a type of machine learning and artificial intelligence that follows the same way humans gain certain types of knowledge. Deep learning is a subset of machine learning. It is called deep learning because it makes use of deep neural networks. Deep learning uses data science, statistics, and predictive modeling to mimic the network of neurons in the human brain and its functioning.
Don’t Miss: What Are The Interview Questions For Data Analyst
What Is Quality Target Product Profile Explain With Examples
The QTPP is defined by capturing all relevant quality requirements of the drug product to be developed.
It consists of following:
i. Dosage form and strength, route of administration, delivery systems, container and closure system etc.
ii. Drug substance quality attributes required for intended drug product, i.e. physical, chemical, and biological properties
iii. Drug product quality attributes required for dosage form, i.e. physical, chemical and microbiological attributes of drug products.
iv. Bioavailability attributes, i.e. dissolution requirement or other relevant characteristics. v. Excipient quality attributes, input material compatibility, stability, pharmacology, etc.
Do You Know How To Use Microsoft Excel Or Similar Spreadsheet Software
Spreadsheet software is a helpful tool for many roles and purposes. Employers ask this question to learn your level of competence when using these programs. When applying to a role that requires spreadsheet experience, it will clearly state this in the job description. In your answer, be prepared to discuss what kind of functions you are familiar with using. If you have never used a spreadsheet before, you may want to communicate that you are a quick learner.
Example:”Yes, I have three years of work experience using Microsoft Excel. I primarily used this software to manage my clients’ information as a salesperson. Using spreadsheets made it easy for me to keep track of my business leads and record their contact information. I also used Microsoft Excel to manage my sales numbers. I found that the equations made it easy to add everything up and predict what numbers I would reach in the future.”
Recommended Reading: How To Study For A Job Interview
Who Should Coordinate The Validation Project
The project coordination of the validation of any system is recommended to be carried out by a person who has the most control and knowledge of the system and the process, for example:
- In the case of ERP systems, it is recommended that the IT area coordinates the validation effort because, it is a system that serves several processes. That would not be practical for process owners to coordinate.
- In the case of LIMS systems for document management, it is recommended that the owner is the person who coordinates the process as they have better control over the process and system requirements.
- For spreadsheets, the same user would be responsible for coordinating and implementing the validation of the leaves.
- If your organization has a PMI or a project office, that would be the responsible entity.
Notwithstanding the above, it should not be forgotten that the validation of computer systems is a joint effort where various stakeholders provide information for the preparation of the elements of the chosen V-model.
It is important that all areas involved support and test the system.
Life cycle
Why Is It Important To Determine The Risks Of The System Before Validation
Primarily to determine the scope of validation, implement controls that reduce or eliminate identified risks to an acceptable level. Knowledge is required to detail the systems risks according to their rank, seniority, complexity and degree of customization, and the process the system serves. The more information you have about it, the better to control risk because we have data on controls performed.
The GAMP® 5 Guide recommends eliminating risk through changes in processes or system design. Design reviews can play a key role in eliminating risk from the start.
Risks that cannot be eliminated from the design must be reduced to an acceptable level by implementing controls. Risk reduction includes applying controls to reduce severity and occurrence and increase detectability.
It should be a systematic approach to ensure that the risk associated with a system has been eliminated or reduced to an acceptable level. The extent of verification and level of detail of documentation should be based on the risk to patient health, product quality and data integrity, especially taking into account the complexity of the system.
It is important to note that risk analysis is part of the risk management system implemented in each company and must be managed as established by each organization.
There are several tools to conduct risk analyses, including: HACCP, HAZOP FMEA. The FMEA tool is recognized by WHO as the most appropriate for the pharmaceutical environment.
You May Like: How To Word A Follow Up Email After Interview
Change Control For Validated Systems
Change Control is a general term describing the process of managing how changes are introduced into a controlled System. Change control demonstrates to regulatory authorities that validated systems remain under control during and after system changes. Change Control systems are a favorite target of regulatory auditors because they vividly demonstrate an organizations capacity to control its systems.
Organizations need to explicitly define their processes for evaluating changes to validated systems. There should be a well defined, multidisciplinary approach to considering the effects from proposed changes. Some changes, such as adding a data field to a form or report may be very minor other changes, such as altering how a program stores and organizes data can be quite extensive. Before changes are implemented, organizations should document the expected outcomes of the changes and have an established plan to implement and test the change and update any existing validation documentation. Part of defining the process for evaluating change control should include the requirements for implementing minor, major and critical changes. This allows the organization to focus proportionate validation resources to the change effort.
Typical Steps in a Change Control project are:
What Good Practices Are Applicable
Among others, the main Good Practices that apply are the following:
- Good Manufacturing Practice is associated with the manufacture of a product, which is produced and controlled to quality standards for use, according to regulations that help ensure reliability to the final consumer.
- Good Laboratory Practice
Read Also: How To Train Managers To Interview
What Is The V
It helps determine the best validation strategy according to the computerized system categorization, specifying the documentation to be generated and the type of tests to be performed at each stage of the validation process. Shows the logic of work in the process of system development and verification.
Determine in advance the V-model to be used so that those involved become familiar with the validation strategy to be followed.
There are several V-models, each suitable for a specific context.
What Are Your Thoughts On Change Control During The Validation Process
An interviewer would ask “What are your thoughts on change control during the validation process?” to a/an Validation Engineer to gain an understanding of the Validation Engineer’s views on how changes should be controlled during the validation process. Change control is important during the validation process because it ensures that changes are made in a controlled and consistent manner, and that the impacts of those changes are properly assessed.
Example: There are a few different schools of thought on change control during the validation process. Some believe that change control is an essential part of the process, and that any changes made to the system under validation should be carefully documented, reviewed, and approved before being implemented. Others believe that change control can be relaxed somewhat during the validation process, since the focus is on ensuring that the system works as intended and not on making changes to it. Ultimately, it is up to the validation team to decide what level of change control is appropriate for their particular project.
Recommended Reading: What Question I Should Ask In Interview
Who Needs Computer System Validation
Computer System Validation is more than a way to avoid profit loss and business risks. In certain instances, it can be life-saving.
For instance, detecting medication defects can prevent unfortunate side effects and avoid significant damage a drug consumer wouldve otherwise received.
Thats why CSV is an obligatory stage for some companies. Heres the list of businesses that are legally enforced to conduct Computer System Validation.
- Pharmaceutical companies. In the US and most developed companies, pharmaceutical businesses have to undergo CSV. In particular, you need to validate your system if your business produces or distributes drugs used for diagnosing or treating diseases.
- Storage and distribution providers. Businesses that store pharmaceuticals, biologicals, or cell-and-tissue products are obliged to complete CSV. Otherwise, the penalties might be as high as debarment or a criminal prosecution.
- Products that sell biologicals. A product can be defined as biological if its made from a therapeutic serum or a virus and used for injury or disease prevention and treatment. Vaccines are biologicals too.
- Medical devices distributors. By definition, medical devices comprise of any instruments used for diagnosis, treatment, or prevention of illnesses. The most common examples of such are lasers, medical implants, tongue depressors, thermometers , and prosthetics.
What Do You Gain From Knowing About The Impact Of Gxp
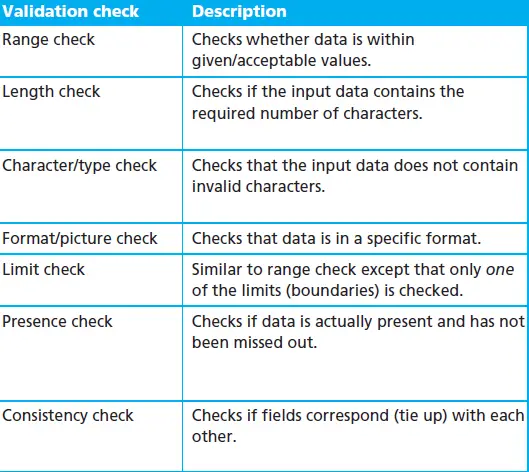
Knowing the impact of GxP allows for a better validation strategy, with particular emphasis on those points where there is an increased risk of negatively impacting the performance of Good Practice also to determine during characterization of the system if it is impact GxP allows differentiate those requiring validation for regulatory compliance of those who do not. The role of process owners and/or Quality System is crucial in this determination to be guardians and empowered the elements of the system under its interference in relation to each regulatory requirement.
In the inventory of computerized systems, the definition of those that have a GxP impact is important. This definition can be provided by considering the following aspects:
Functionality of systems with a GxP impact:
1. The creation, maintenance or preservation of records or documentation required by the Good Practice regulations for evaluating product quality and making security decisions.
2. Automation of Best Practices, product quality or product safety decisions.
3. The data output to other system modules or external systems with the above features.
4. The input process data from other system modules or other systems with the above features.
It is also important to generate evidence of why some systems are not considered GxP compliant. This evidence can be provided during system characterization using a checklist.
Also Check: Java Collections Coding Interview Questions For 5 Years Experience